🧠 How to Select Injection Molding Materials Complying with ISO 13485?
Ensure Your Medical Device Materials Are Compliant, Reliable, and Safe
When it comes to medical device manufacturing, choosing the right injection molding material is far more than a matter of mechanical performance. Under ISO 13485, material selection becomes a regulated, risk-managed, and fully traceable process. At TXS, we help global medical brands navigate this process with confidence and precision.
🔍 Why ISO 13485 Material Compliance Matters
The ISO 13485:2016 standard ensures the quality and safety of medical devices throughout their lifecycle. It places strict requirements on:
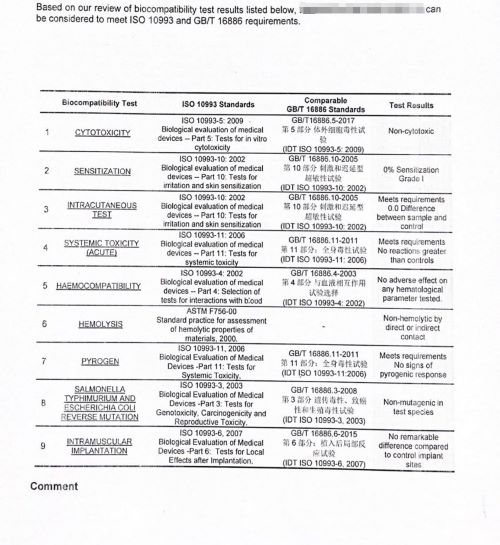
- ✅ Biocompatibility (e.g., ISO 10993 testing)
- ✅ Sterilization compatibility (e.g., EO, gamma, steam)
- ✅ Traceability of every raw material batch
- ✅ Risk mitigation for contamination, material degradation, and regulatory non-compliance
📌 Non-compliant materials can lead to:
❌ Device failure
❌ Regulatory penalties
❌ Delayed product launch
❌ Patient safety issues
📘 ISO 13485 Requirements for Injection Molding Materials
ISO 13485 doesn’t list specific materials but defines process controls and documentation protocols. Key requirements include:
1. 🔍 Supplier Qualification
- Materials must come from audited or certified suppliers (ISO 13485, ISO 9001)
- TXS maintains a qualified vendor system and performs regular supplier assessments
2. 📋 Regulatory Documentation
- Support for ISO 10993, USP Class VI, and FDA Master File inclusion
- TXS provides compliance certificates, test reports, and validation data for all materials
3. ⚠️ Risk-Based Selection
- Evaluate risk factors: sterilization degradation, cytotoxicity, or contamination
- TXS supports material simulation and stress-testing to prevent failure in use
4. 🔗 Full Traceability
- Lot numbers, test reports, COAs, and material origin must be recorded and traceable
- Especially essential for Class II/III implantable or IVD devices
5. 🔄 Change Control
- Any change in material (brand, batch, grade) must go through formal change validation
- TXS supports design history file (DHF) and product risk documentation
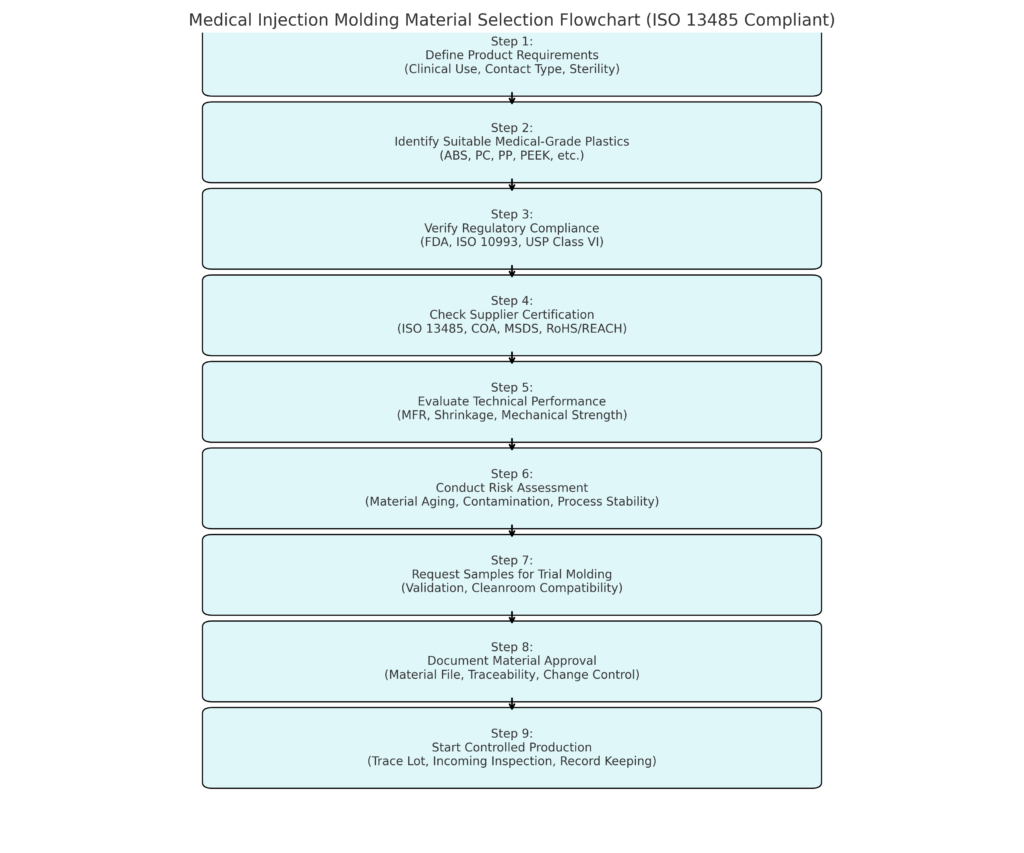
✅ ISO 13485-Compliant Material Selection Flowchart
We’ve developed a practical 9-step workflow to guide your material selection under ISO 13485:
📊 Flowchart: ISO 13485 Injection Molding Material Selection
🧩 Common Medical-Grade Plastics & Their Applications
| Material | Key Benefits | Applications |
|---|---|---|
| PP (Polypropylene) | Sterilizable, fatigue-resistant | Syringes, disposable components |
| PC (Polycarbonate) | High impact strength, clear, EO resistant | Device housings, IVD cartridges |
| PEEK | Biocompatible, high-temp, implant-grade | Surgical guides, orthopedic anchors |
| ABS | Flame-retardant, moldable, cost-effective | Monitors, enclosures, pump covers |
🔬 TXS provides ISO 10993 & USP Class VI material test support on request.
🏭 Why Choose TXS for Medical-Grade Injection Molding?
At TXS, we combine compliance and innovation to deliver full-spectrum medical manufacturing support:
-
🧪 100,000-class clean room & 10,000-class microbiological lab
-
🛠️ ISO 13485-certified production system with traceability tools
-
📦 End-to-end service: material → mold → molding → assembly → packaging
🌍 Serving clients across the U.S., Japan, Canada, Europe, and the Middle East
✉️ Let’s Engineer Your Medical Product Together
Whether you're launching a new medical device or optimizing an existing one, our team of technical engineers and compliance experts can help you select the best-fit material under ISO 13485.
📩 Contact us today:
📧 [email protected]
🌐 www.molds-maker.com